Abstract
Introduction: L-glutamine plays an important role in regulating oxidative stress, one of the key contributors to sickle cell disease pathophysiology. An earlier 48-week Phase 3 study conducted in the United States demonstrated significant reductions in acute complications associated with sickle cell disease, such as vaso-occlusive crises (VOCs) and acute chest syndrome (ACS) in patients on L-glutamine therapy compared to those on placebo (Niihara et al., 2018). This study also showed a significantly fewer number of hospitalizations and hospitalization days for those treated with L-glutamine. Our current real-world observational study is the first to report long term evaluation (120 weeks) of efficacy and safety of L-glutamine (Endari®) therapy in patients living with sickle cell disease in Qatar and French Guiana.
Methods: Nineteen patients (10 pediatric and 9 adults), 63% on hydroxyurea, with confirmed diagnosis of sickle cell disease (HbSS genotype) were treated with L-glutamine oral powder (~0.3 g/kg body weight) twice daily for 120 weeks. Of the enrolled patients, 4 patients were from Qatar with the Arab-Indian haplotype and 15 patients were from French Guiana with the African haplotype. The median age was 17 years (range 8 to 54 years) where 53% were <18 years of age. The median body weight of patients was 50 kilograms (range of 25 to 75 kilograms); 53% were males. Clinical events and laboratory parameters (hemoglobin, hematocrit, reticulocytes, and LDH) were collected or measured at baseline, 24, 48, 72, 96, and at 120 weeks. Baseline measures for VOCs, number of hospitalizations, hospitalization days, ACS events, and blood transfusions were collected for the 12 months prior to L-glutamine initiation. Changes from baseline to 120 weeks (annualized) were analysed using MedCalc statistical software Version 20.015.
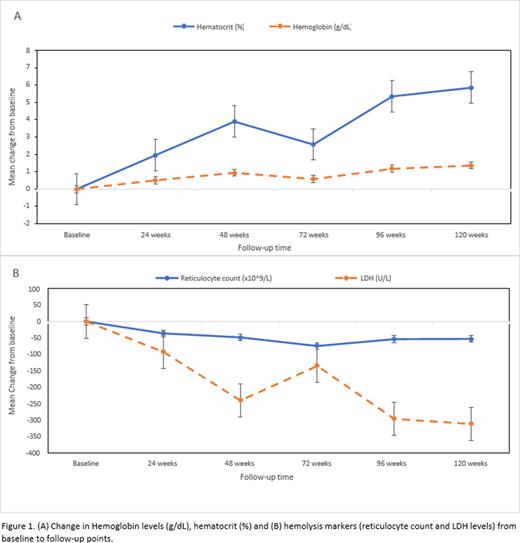
Results: Eighteen patients completed 120 weeks of the real-world L-glutamine treatment observation period. There were significantly fewer VOCs; median of 3 VOCs at baseline and median of 0 at 120 weeks. There were significantly fewer hospitalizations (median of 3 hospitalizations at baseline and 0 at 120 weeks), shorter hospitalization days (median of 15 days at baseline and 0 at end of the observation period), and fewer blood transfusions (median of 3 at baseline and 0 at 120 weeks). The change from baseline values to 120 weeks were significant (p < .00001) for VOCs, hospitalizations, days hospitalized, and the number of units transfused. The number of ACS events were also reduced from 11 at baseline to 0 at 120 weeks. Consistent with these findings, the mean increase in hemoglobin concentrations from baseline was 1.36 g/dL ± 0.17 SE and the mean percent increase in hematocrit from baseline was 5.85% ± 1.10 SE, both significantly improved at 120 weeks (p < .001) Figure 1A. Furthermore, mean reticulocyte counts decreased from baseline (-52.39 x 109/L ± 25.68 SE) and LDH levels decreased from baseline (-311.11 U/L ± 56.02 SE) both significantly changed at 120 weeks (p < .001) Figure 1B. Fewer number of patients were taking hydroxyurea at 120 weeks (8 patients) compared to baseline (12 patients). One patient died during the study (after the 72-week follow up visit) due to multiple deep vein thromboses resulting in a pulmonary embolism; this event was not related to L-glutamine treatment.
Conclusion: In this long-term observational study, oral L-glutamine therapy in pediatric and adults living with sickle cell disease resulted in sustained clinical efficacy with improvements in hematologic parameters. L-glutamine therapy was well-tolerated by all patients and there were no safety concerns.
Disclosures
Elenga:Addmedica: Honoraria. Etienne-Julan:Addmedica: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal